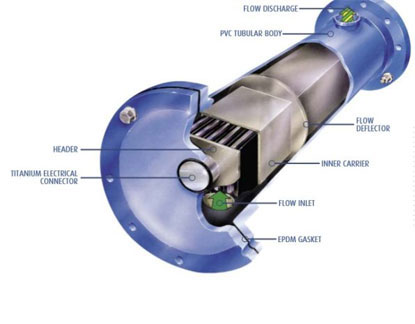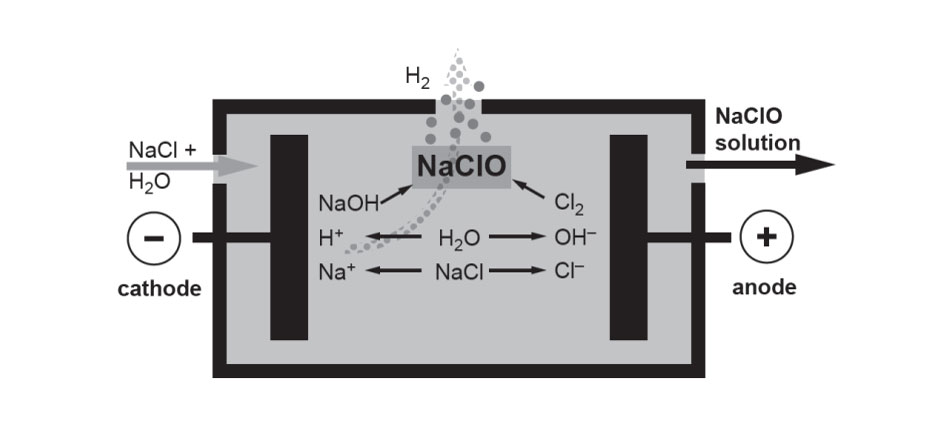
The electrolysis of seawater or artificial brine in an electrochemical cell produces sodium hypochlorite by a combination of electrochemical and chemical reactions. At the anode the oxidation of chloride ions to produce chlorine takes place:
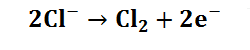
At the Cathode the reduction of water to produce hydrogen takes place:
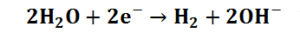
The electrolysis takes place in an undivided cell with a slightly alkaline pH so that as soon as chlorine is liberated at the anode it immediately reacts with water to produce hypochlorite:

The overall process therefore can be summarised as the reaction of salt with water according to:
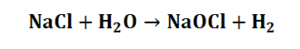
Hypochlorite is a powerful biocide and although it eventually decomposes back to chloride ions and oxygen it has the advantage over other biocides in that it is relatively stable. Electrolytic production of hypochlorite satisfies the instantaneous requirement for biocide.